Elements in group and period 2021/Best Element Chapter/Periods Table 2021/ CBSE 10th Science Board Exam 2021: Important MCQs from Chapter 5 Periodic Classification of Elements/what is a group on the periodic table/periodic table groups 1-8/periodic table with names/periodic table group names 1-18/periodic table groups and periods/group of elements/118 elements and their symbols/atomic number of elements from 1 to 30/atomic number list/periodic table of elements with names and symbols
Elements in groups and periods
There are just about 120 known components. For instance, you are comprised of billions of billions of particles yet you .
Elements
CHEMICAL ELEMENTS ARE those substances that cannot be split
up into other substances. For example, gold cannot be split but water can be
split into the elements hydrogen and oxygen. Each element is made up of atoms with
fixed number of protons in their nucleus. Atoms of gold have 79 protons in
their nucleus while atoms of hydrogen have 1.-Elements in groups and periods
Number of elements
There are about 85 naturally occurring elements. But scientists
have discovered about 25 more, making the total number of identified Elements in groups and periods is ,
112.
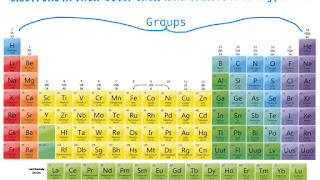
Periodic table
Periodic table is a chat devised by John Dalton (1766-1844)
on which all the elements can be arranged according to the number of protons in
the nucleus. The columns in the table are called Groups, and the rows are
called Periods. Each period contains elements that have atoms with the same
number of electron shells. In each period, the number of protons along With the
number of electrons in the atom increases one by one. Elements in the same Group
have similar properties, because they have the same number of electrons in the
Outer shell of their atoms.
The Periodic Table
The Periodic Table arranges all the elements in Periods
(rows) and Groups (columns). The colors show some of the major kinds of
elements, including metals and transition elements.
What are the noble gases?
Noble gases are the elements listed in Group 0, located on
the far right of the Table. The outer electron shells in these have a full
complement of electrons. That is why they rarely react with other substances.
They are therefore called inert gases also. Argon and xenon are noble gases
that are often used in light bulbs, because the filament in the light bulb
heats up without burning.
Reactive elements
If elements gain or lose electrons readily they are reactive.
The further to the left they are in the Table, the more reactive they are.
Group 1 metals, such as sodium and potassium are very reactive.
What is metal?
Metals are those substances that are dense, hard, and shiny that
ring when they are hit with another metal. Also, metals are good conductors of
heat and electricity.
Compounds
Compounds are substances that are formed when two or more
elements are combined. In a compound, each molecule (smallest particle) has the
same combination of atoms. For example, molecules of sodium chloride are one
sodium atom joined to one chlorine atom. The properties of a compound are
different from the properties of the elements that form the compound. Sodium
splits when put in water; chlorine is a thick green gas, yet sodium chloride is
table salt.
About The Post
#what is a group on the periodic table

Comments
Post a Comment